Morphology-, synthesis- and doping-independent tuning of ZnO work function using phenylphosphonates†
Abstract
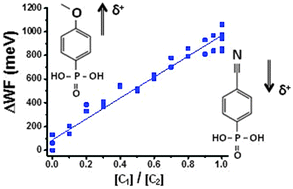
The work function (WF) of ZnO is modified by two types of dipole-bearing phenylphosphonate layers, yielding a maximum WF span of 1.2 eV. H3CO-phenyl phosphonate, with a positive dipole (positive pole pointing outwards from the surface), lowers the WF by ∼350 meV. NC-phenyl phosphonate, with a negative dipole, increases the WF by ∼750 meV. The WF shift is found to be independent of the type of ZnO surface. XPS data show strong molecular dipoles between the phenyl and the functionalizing (CN and OMe) tail groups, while an opposite dipole evolves in each molecular layer between the surface and the phenyl rings. The molecular modification is found to be invariant to supra-bandgap illumination, which indicates that the substrate's space charge-induced built-in potential is unlikely to be the reason for the WF difference. ZnO, grown by several different methods, with different degrees of crystalline perfection and various morphologies and crystallite dimensions, could all be modified to the same extent. Furthermore, a mixture of opposite dipoles allows gradual and continuous tuning of the WF, varying linearly with the partial concentration of the CN-terminated phosphonate in the solution. Exposure to the phosphonic acids during the molecular layer deposition process erodes a few atomic layers of the ZnO. The general validity of the treatment and the fine-tuning of the WF of treated interfaces are of interest for solar cells and LED applications.

 Please wait while we load your content...
Please wait while we load your content...