The role of charge transfer in the energy level alignment at the pentacene/C60 interface
Abstract
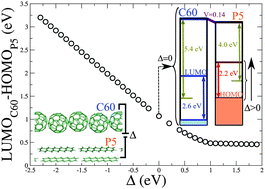
Understanding the mechanism of energy level alignment at organic–organic interfaces is a crucial line of research to optimize applications in organic electronics. We address this problem for the C60–pentacene interface by performing local-orbital Density Functional Theory (DFT) calculations, including the effect of the charging energies on the energy gap of both organic materials. The results are analyzed within the induced density of interface states (IDIS) model. We find that the induced interface potential is in the range of 0.06–0.10 eV, in good agreement with the experimental evidence, and that such potential is mainly induced by the small, but non-negligible, charge transfer between the two compounds and the multipolar contribution associated with pentacene. We also suggest that an appropriate external intercompound potential could create an insulator–metal transition at the interface.

 Please wait while we load your content...
Please wait while we load your content...