Dissociative electron attachment to gas phase thiothymine: experimental and theoretical approaches
Abstract
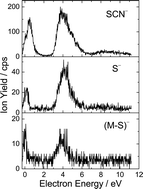
In this contribution we have investigated experimentally and theoretically the interaction of low energy electrons with gas phase thiothymine (a sulphur containing analogue of thymine). We observe that the presence of the sulphur atom within thiothymine strongly controls the fragmentation dynamics. With the exception of the (M − H)− anion formation, the most favorable reaction channels are associated with a loss of sulphur containing negative fragments (i.e., the formation of S−, SCN− and (M − S)−) suggesting that these resonances are localized at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S group. Hence the present results demonstrate that certain reactions can be controlled by substitution of the sulphur atom at specific molecular sites within nucleobases. Our study thus represents a starting point for a physicochemical understanding of the action of sulphur-containing antimetabolites when used in chemoradiotherapy.
S group. Hence the present results demonstrate that certain reactions can be controlled by substitution of the sulphur atom at specific molecular sites within nucleobases. Our study thus represents a starting point for a physicochemical understanding of the action of sulphur-containing antimetabolites when used in chemoradiotherapy.

 Please wait while we load your content...
Please wait while we load your content...