Aggregation induced emission enhancement from Bathophenanthroline microstructures and its potential use as sensor of mercury ions in water
Abstract
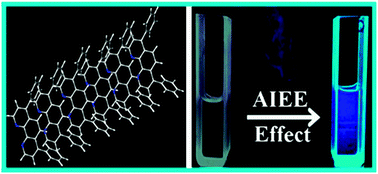
Bathophenanthroline (BA) microstructures of various morphologies have been synthesized using a reprecipitation method. The morphologies of the particles are characterized using optical and scanning electron microscopy (SEM) methods. An aqueous dispersion of BA microstructures shows aggregation induced emission enhancement (AIEE) compared to BA in a good solvent, THF. This luminescent property of aggregated BA hydrosol is used for the selective detection of trace amounts of mercury ion (Hg2+) in water. It is observed that Hg2+ ions can quench the photoluminescence (PL) intensity of BA aggregates even at very low concentrations, compared to other heavy metal ions e.g. nickel (Ni2+), manganese (Mn2+), cadmium (Cd2+), cobalt (Co2+), copper (Cu2+), ferrous (Fe2+) and zinc (Zn2+). This strong fluorescence quenching of aggregated BA in the presence of Hg2+ ions has been explained as a complex interplay between the ground state complexation between BA and Hg2+ ions and external heavy atom induced perturbation by Hg2+ ions on the excited states of the fluorophore BA.

 Please wait while we load your content...
Please wait while we load your content...