On the theory of high rate capability of LiMn2O4 with some preferred orientations: insights from the crystal shape algorithm
Abstract
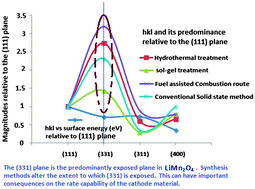
The predominant orientation of LiMn2O4 synthesized through the different methods is attributed, using the crystal shape algorithm (a new tool advanced to study the crystal shapes of crystalline materials), to the (331) plane. Existing literature evidence however shows that the (400) plane is the thermodynamically most stable hkl direction of LiMn2O4. Observations from the crystal shape algorithm and literature evidence of the thermodynamic stabilities of the hkl planes of LiMn2O4 point to the operation of a kinetically controlled mechanism governing the LiMn2O4 synthesis reactions currently available in the literature. This finding can have important consequences on the electrochemical characteristics of the material such as its rate capability.

 Please wait while we load your content...
Please wait while we load your content...