Kinetics of CH2OO reactions with SO2, NO2, NO, H2O and CH3CHO as a function of pressure†
Abstract
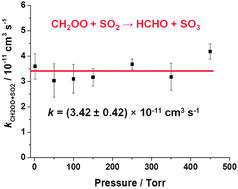
Kinetics of CH2OO Criegee intermediate reactions with SO2, NO2, NO, H2O and CH3CHO and CH2I radical reactions with NO2 are reported as a function of pressure at 295 K. Measurements were made under pseudo-first-order conditions using flash photolysis of CH2I2–O2–N2 gas mixtures in the presence of excess co-reagent combined with monitoring of HCHO reaction products by laser-induced fluorescence (LIF) spectroscopy and, for the reaction with SO2, direct detection of CH2OO by photoionisation mass spectrometry (PIMS). Rate coefficients for CH2OO + SO2 and CH2OO + NO2 are independent of pressure in the ranges studied and are (3.42 ± 0.42) × 10−11 cm3 s−1 (measured between 1.5 and 450 Torr) and (1.5 ± 0.5) × 10−12 cm3 s−1 (measured between 25 and 300 Torr), respectively. The rate coefficient for CH2OO + CH3CHO is pressure dependent, with the yield of HCHO decreasing with increasing pressure. Upper limits of 2 × 10−13 cm3 s−1 and 9 × 10−17 cm3 s−1 are placed on the rate coefficients for CH2OO + NO and CH2OO + H2O, respectively. The upper limit for the rate coefficient for CH2OO + H2O is significantly lower than has been reported previously, with consequences for modelling of atmospheric impacts of CH2OO chemistry.

 Please wait while we load your content...
Please wait while we load your content...