The structure of hyperalkaline aqueous solutions containing high concentrations of gallium – a solution X-ray diffraction and computational study
Abstract
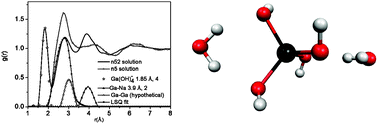
Highly concentrated alkaline NaOH–Ga(OH)3 solutions with 1.18 M ≤ [Ga(III)]T ≤ 2.32 M and 2.4 M ≤ [NaOH]T ≤ 4.9 M (where the subscript T denotes total or analytical concentrations) have been prepared and investigated by solution X-ray diffraction and also by ab initio quantum chemical calculations. The data obtained are consistent with the presence of only one predominant Ga(III)-bearing species in these solutions, which is the tetrahedral hydroxo complex Ga(OH)4−. This finding is in stark contrast to that found for Al(III)-containing solutions of similar concentrations, in which, besides the monomeric complex, an oxo-bridged dimer was also found to form. From the solution X-ray diffraction measurements, the formation of the dimeric (OH)3Ga–O–Ga(OH)32− could not unambiguously be shown, however, from the comparison of experimental IR, Raman and 71Ga NMR spectra with calculated ones, its formation can be safely excluded. Moreover, higher mononuclear stepwise hydroxo complexes, like Ga(OH)63−, which have been claimed to exist by others in the literature, were not possible to experimentally detect in these solutions with any of the spectroscopic techniques used.

 Please wait while we load your content...
Please wait while we load your content...