Experimental and theoretical study of the electronic properties of Cu-doped anatase TiO2
Abstract
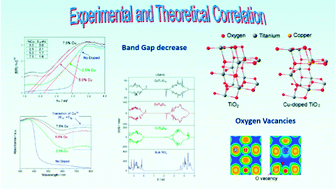
A good correlation was obtained between the electronic properties of Cu-doped anatase TiO2 by virtue of both physical chemistry characterization and theoretical calculations. Pure and Cu-doped TiO2 were synthesized. The composition, structural and electronic properties, and the band gap energy were obtained using several techniques. The method of synthesis used produces Cu-doped anatase TiO2, and XRD, XPS and Raman spectroscopy indicate that Cu atoms are incorporated in the structure by substitution of Ti atoms, generating a distortion of the structure and oxygen vacancies. In turn, the band gap energy of the synthesized samples decrease drastically with the Cu doping. Moreover, periodic density functional theory (DFT-periodic) calculations were carried out both to model the experimentally observed doped structures and to understand theoretically the experimental structures obtained, the formation of oxygen vacancies and the values of the band gap energy. From the analysis of density of states (DOS), projected density of states (PDOS) and the electron localization function (ELF) a decrease in the band gap is predicted upon increasing the Cu doping. Thus, the inclusion of Cu in the anatase structure implies a covalent character in the Cu–O interaction, which involves the appearance of new states in the valence band maximum with a narrowing in the band gap.

 Please wait while we load your content...
Please wait while we load your content...