A spectroscopic investigation and molecular docking study on the interaction of hen egg white lysozyme with liposomes of saturated and unsaturated phosphocholines probed by an anticancer drug ellipticine
Abstract
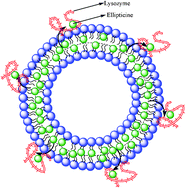
Interaction of hen egg white lysozyme with different liposomes made of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 2-oleoyl-1-palmitoyl-sn-glycero-3-phosphocholine (POPC) was studied by circular dichroism (CD), steady state and time resolved fluorescence spectroscopy. We used anticancer drug ellipticine and studied its entrapment and release from liposomes upon interaction with lysozyme. The molecular docking study revealed that ellipticine preferably binds to the hydrophobic pocket of lysozyme (Kbinding = 1.09 × 106 M−1). The binding was also supported by spectroscopic evidence. Addition of lysozyme to the ellipticine impregnated liposomes caused quenching of the fluorescence intensity of ellipticine as lysozyme induces hydration and phospholipid rearrangement in the bilayers leading to the leakage of drug molecules. The extent of quenching depends on the prehydration level of liposomes. Maximum quenching took place in the DPPC liposome as it is the least hydrated while minimum quenching was observed in the DOPC liposome having the highest hydration level among all the lipids. The time resolved studies revealed that both the fast and slow lifetime components of ellipticine decrease significantly with addition of lysozyme. This fact is attributed to lysozyme induced hydration and rupture of bilayers. It is revealed that upon addition of lysozyme to liposomes, the amplitude of the fast component increases and that of the slow component decreases which imply that the drug molecules are released from liposomes and subsequent migration takes place to the aqueous phase. Molecular docking studies and fluorescence measurements indicate that ellipticine after removal from the liposome binds to the hydrophobic binding site of lysozyme.

 Please wait while we load your content...
Please wait while we load your content...