Ion effects at electrode/solid polymer electrolyte membrane interfaces
Abstract
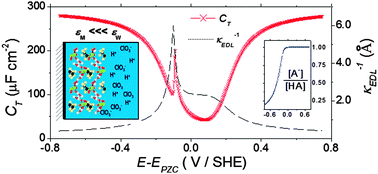
The differential capacity and the potential distribution at electrode/solid polymer electrolyte membrane/solution interfaces are calculated through an analytical approach. The model considers coions' and counterions' permeation through the membrane from the solvent phase and the ions' partitioning equilibrium at the SPEM/solution interface. The latter effects are included by incorporating the Donnan equilibrium, the steric hindrance, the solvation energy change when ions move from water to membrane pores and ion electrostatic interactions. It is shown that capacitance maxima in capacitance–potential curves may appear because of the acid–base dissociation process inside the membrane and the change in the ions' total interaction energy with the applied potential. For low dielectric constants inside membrane pores, εp, sharp peaks can be obtained. These peaks broaden, decrease in magnitude and shift to positive potentials once εp is increased. Finally, model predictions are discussed in light of recent experimental data obtained on Nafion® covered Pt(111) electrodes, providing a theoretical framework for the qualitative electroanalysis of these systems.

 Please wait while we load your content...
Please wait while we load your content...