Electron density shape analysis of a family of through-space and through-bond interactions
Abstract
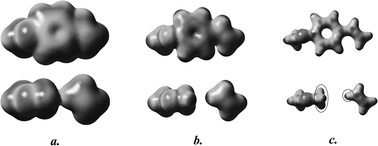
A family of styrene derivatives has been used to study the effects of through-space and through-bond interactions on the local and global shapes of electron densities of complete molecules and a set of substituents on their central rings. Shape analysis methods which have been used extensively in the past for the study of molecular property–molecular shape correlations have shown that in these molecules a complementary role is played by the through-space and through-bond interactions. For each specific example, the dominance of either one of the two interactions can be identified and interpreted in terms of local shapes and the typical reactivities of the various substituents. Three levels of quantum chemical computational methods have been applied for these structures, including the B3LYP/cc-pVTZ level of density functional methodology, and the essential conclusions are the same for all three levels. The general approach is suggested as a tool for the identification of specific interaction types which are able to modify molecular electron densities. By separately influencing the through-space and through-bond components using polar groups and groups capable of conjugation, some fine-tuning of the overall effects becomes possible. The method described may contribute to an improved understanding and control of molecular properties involving complex interactions with a possible role in the emerging field of molecular design.

 Please wait while we load your content...
Please wait while we load your content...