Probing the electronic and geometric structure of ferric and ferrous myoglobins in physiological solutions by Fe K-edge absorption spectroscopy†
Abstract
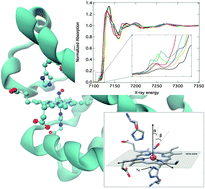
We present an iron K-edge X-ray absorption study of carboxymyoglobin (MbCO), nitrosylmyoglobin (MbNO), oxymyoglobin (MbO2), cyanomyoglobin (MbCN), aquomet myoglobin (metMb) and unligated myoglobin (deoxyMb) in physiological media. The analysis of the XANES region is performed using the full-multiple scattering formalism, implemented within the MXAN package. This reveals trends within the heme structure, absent from previous crystallographic and X-ray absorption analysis. In particular, the iron–nitrogen bond lengths in the porphyrin ring converge to a common value of about 2 Å, except for deoxyMb whose bigger value is due to the doming of the heme. The trends of the Fe–Nε (His93) bond length is found to be consistent with the effect of ligand binding to the iron, with the exception of MbNO, which is explained in terms of the repulsive trans effect. We derive a high resolution description of the relative geometry of the ligands with respect to the heme and quantify the magnitude of the heme doming in the deoxyMb form. Finally, time-dependent density functional theory is used to simulate the pre-edge spectra and is found to be in good agreement with the experiment. The XAS spectra typically exhibit one pre-edge feature which arises from transitions into the unoccupied dσ and dπ − πligand* orbitals. 1s → dπ transitions contribute weakly for MbO2, metMb and deoxyMb. However, despite this strong Fe d contribution these transitions are found to be dominated by the dipole (1s → 4p) moment due to the low symmetry of the heme environment.

 Please wait while we load your content...
Please wait while we load your content...