Constructing (super)alkali–boron-heterofullerene dyads: an effective approach to achieve large first hyperpolarizabilities and high stabilities in M3O–BC59 (M = Li, Na and K) and K@n-BC59 (n = 5 and 6)†
Abstract
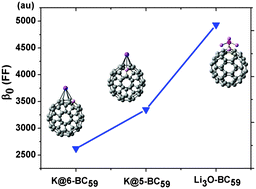
Using DFT methods, the electronic properties and the first hyperpolarizabilities of boron-heterofullerene–(super)alkali dyads: M3O–BC59 (M = Li, Na and K) and K@n-BC59 (n = 5 and 6) were systematically investigated. It is found that both M3O and K can effectively bind to BC59 with high binding energies (2.50–2.69 eV for K and 4.24–5.14 eV for M3O). The interaction between K and BC59 in K@n-BC59 is identified as primarily ionic in nature, whereas that between the superalkali M3O unit and BC59 becomes much stronger owing to the formation of a strong chemical bond (B–O bond). Moreover, compared with the sole parent cluster BC59 (619 au), both K@n-BC59 (n = 5 and 6) and M3O–BC59 (M = Li, Na and K), possess large first hyperpolarizabilities (β0), which are 3352, 2621 and 4921, 5440 and 7800 au, respectively, where the superalkali doped dyads (M3O–BC59) are much superior to the simple alkali exo-hedral species (K@n-BC59), and heavier superalkali can be more powerful in enhancing the β0 values of M3O–BC59. Clearly, these superalkali doped dyads M3O–BC59, formal donor–acceptor (DA) chromophores, exhibit not only excellent stability but also large first hyperpolarizability; therefore, they are expected to be potential candidates for excellent second-order NLO materials.

 Please wait while we load your content...
Please wait while we load your content...