Oxygen adsorption on single layer graphyne: a DFT study†
Abstract
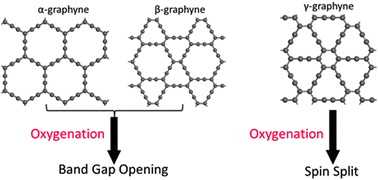
Graphyne is a rising two-dimensional (2D) carbon allotrope with excellent electronic properties. In this paper, theoretical calculations were performed to study the corresponding electronic properties of the oxygenated graphyne. Atomic oxygen when bound to the carbon atom of graphyne forms a stable oxide, with a much larger binding energy compared to that on graphene. Owing to the oxygen adsorption, the α- and β-graphyne change from a zero-band-gap material to a semiconductor as indicated in the band structure calculations. Moreover, spin splitting was observed from the band structure of the oxygenated γ-graphyne. These electronic properties are tunable by altering the oxygen coverage through changing the supercell size. Our results based on the first-principles calculations imply that oxygenation is a promising method to functionalize graphyne to achieve designated properties.

 Please wait while we load your content...
Please wait while we load your content...