Approach to multi-electron reduction beyond two-electron reduction of CO2
Abstract
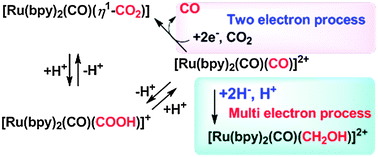
Dicationic [Ru(bpy)2(CO)2]2+ (bpy = 2,2′-bipyridyl) exists as an equilibrium mixture of [Ru(bpy)2(CO)(COOH)]+ and [Ru(bpy)2(CO)(CO2)] in H2O. Photo- and electrochemical reduction of [Ru(bpy)2(CO)2]2+ in the presence of proton sources under CO2 produced CO and HCOOH with generation of [Ru(bpy)2(CO)(CO2)], which spontaneously comes to an equilibrium of [Ru(bpy)2(CO)(COOH)]+ and [Ru(bpy)2(CO)2]2+. Two-electron reduction of CO2, giving CO and/or HCOOH depending on the proton concentration, is ascribed to reductive cleavage of M–COOH and M–CO bonds derived from M–CO2 complexes. Catalytic DMF production in the electrochemical reduction of [Ru(bpy)2(CO)2]2+ in the presence of Me2NH under CO2 is also explained by reductive cleavage of the Ru–C(O)NMe2 bond. On the other hand, the treatment of [Ru(bpy)2(CO)2]2+ with BH4− produced CH3OH via [Ru(bpy)2(CO)(CHO)]+ and [Ru(bpy)2(CO)(CH2OH)]+, indicating that reduction of M–CO complexes with renewable hydride donors is a feasible pathway to realize catalytic six-electron reduction of CO2 affording CH3OH. Along these lines, [Ru(bpy)2(pbn)]2+ (pbn = 2-(2-pyridyl)benzo[b]-1,5-naphthyridine) was prepared to simulate the biological role of the NAD/NADH redox couple. Irradiation of visible light on[Ru(bpy)2(pbn)]2+ in the presence of a sacrificial electron donor produced smoothly the two-electron reduced form, [Ru(bpy)2(pbnHH)]2+ in a quantum yield of 20%. Furthermore, hydride transfer from [Ru(bpy)2(pbnHH)]2+ to CO2 took place in the presence of base to regenerate [Ru(bpy)2(pbn)]2+ accompanied by HCOO− formation.

 Please wait while we load your content...
Please wait while we load your content...