Correlating cation ordering and voltage fade in a lithium–manganese-rich lithium-ion battery cathode oxide: a joint magnetic susceptibility and TEM study†
Abstract
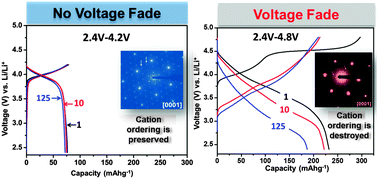
Structure–electrochemical property correlation is presented for lithium–manganese-rich layered–layered nickel manganese cobalt oxide (LMR–NMC) having composition Li1.2Co0.1Mn0.55Ni0.15O2 (TODA HE5050) in order to examine the possible reasons for voltage fade during short-to-mid-term electrochemical cycling. The Li1.2Co0.1Mn0.55Ni0.15O2 based cathodes were cycled at two different upper cutoff voltages (UCV), 4.2 V and 4.8 V, for 1, 10, and 125 cycles; voltage fade was observed after 10 and 125 cycles only when the UCV was 4.8 V. Magnetic susceptibility and selected-area electron diffraction data showed the presence of cation ordering in the pristine material, which remained after 125 cycles when the UCV was 4.2 V. When cycled at 4.8 V, the magnetic susceptibility results showed the suppression of cation ordering after one cycle; the cation ordering diminished upon further cycling and was not observed after 125 cycles. Selected-area electron diffraction data from oxides oriented towards the [0001] zone axis revealed a decrease in the intensity of cation-ordering reflections after one cycle and an introduction of spinel-type reflections after 10 cycles at 4.8 V; after 125 cycles, only the spinel-type reflections and the fundamental O3 layered oxide reflections were observed. A significant decrease in the effective magnetic moment of the compound after one cycle at 4.8 V indicated the presence of lithium and/or oxygen vacancies; analysis showed a reduction of Mn4+ (high spin/low spin) in the pristine oxide to Mn3+ (low spin) after one cycle. The effective magnetic moment was higher after 10 and 125 cycles at 4.8 V, suggesting the presence of Mn3+ in a high spin state, which is believed to originate from distorted spinel (Li2Mn2O4) and/or spinel (LiMn2O4) compounds. The increase in effective magnetic moments was not observed when the oxide was cycled at 4.2 V, indicating the stability of the structure under these conditions. This study shows that structural rearrangements in the LMR–NMC oxide happen only at higher potentials (4.8 V, for example) and provides evidence of a direct correlation between cation ordering and voltage fade.

 Please wait while we load your content...
Please wait while we load your content...