Theoretical investigation of porphyrin-based photosensitizers with enhanced NIR absorption
Abstract
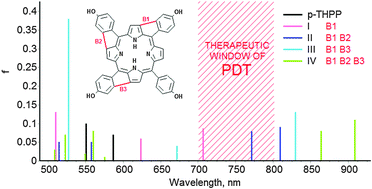
Porphyrins are compounds which are fundamental for designing many photosensitizers assigned for use in photodynamic therapy (PDT). However, photosensitizers available on the drug market are not ideal for use in PDT among others because of their low absorption in the wavelength range optimal for tissue penetration, i.e. the near-infrared (NIR) spectral region. In the present study the density functional theory and its time dependent extension calculations have been used to design new porphyrin-based photosensitizers with absorption in the therapeutic window of PDT of 700–800 nm. Theoretical investigation of four different derivatives of the 5,10,15,20-tetrakis(4-hydroxyphenyl)porphyrin (p-THPP) revealed specific ‘bridge’ configurations which have a significant influence on absorption spectra. The results of the present study may be a useful starting point for future design of porphyrin derivatives as improved photosensitizers.

 Please wait while we load your content...
Please wait while we load your content...