Bond and mode selectivity in the OH + NH2D reaction: a quasi-classical trajectory calculation
Abstract
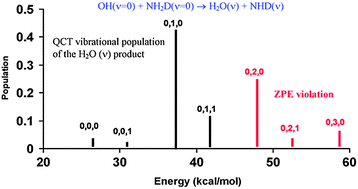
A state-to-state dynamics study was performed to analyze the effects of vibrational excitation on the dynamics of the OH + NH2D gas-phase reaction, which are connected to issues such as bond and mode selectivity. This reaction can evolve along two channels: H-abstraction, H2O(ν) + NHD(ν); and D-abstraction, HOD(ν) + NH2(ν). Based on an analytical potential energy surface previously developed by our group, quasi-classical trajectory calculations and subsequent normal mode analysis were performed. While vibrational excitation of the NH-sym mode of NH2D slightly favours H-abstraction over the D-abstraction, vibrational excitation of the ND mode shows that there is no clear preference for the H- or D-abstraction. These results show that this reaction does not exhibit bond selectivity, suggesting a breakdown of the spectator model. For H-abstraction, vibrational excitation of the non-reactive ND mode is partially retained in the NHD product; and for D-abstraction, excitation of the non-reactive NH mode is also partially retained in the products, indicating that this reaction exhibits mode selectivity only partially. In sum, we rule out bond and mode selectivity for this reaction. All these results were interpreted on the basis of strong coupling between modes along the reaction path, a behaviour which seems to be more the general tendency than the exception in polyatomic reactions.

 Please wait while we load your content...
Please wait while we load your content...