Ligand exchange in ionic systems and its effect on silver nucleation and growth
Abstract
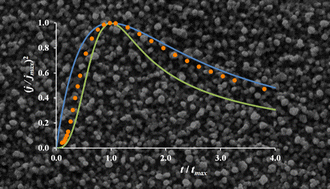
The electrodeposition of metals from ionic solutions is intrinsically linked to the reactivity of the solute ions. When metal salts dissolve, the exchange of the anion with the molecular and ionic components from solution affects the speciation and therefore the characteristics of metal reduction. This study investigates the nucleation mechanism, deposition kinetics, metal speciation and diffusion coefficients of silver salts dissolved in Deep Eutectic Solvents. The electrochemical reduction of AgCl, AgNO3 and Ag2O is studied in 1 : 2 choline chloride : ethylene glycol and 1 : 2 choline chloride : urea. Cyclic voltammetry is used to evaluate electrochemical kinetics. Detailed analysis of chronoamperometric data shows that silver deposits form via multiple 3D nucleation with mass transport controlled hemispherical growth. The nucleation mechanism was found to be potential dependent, varying from progressive to instantaneous as the reduction potential becomes more cathodic. Diffusion coefficients are determined using three different methods. Trends are rationalised in terms of solvent viscosity and silver speciation analysis with EXAFS. The morphology of electroreduced silver is investigated with scanning electron microscopy and shows that deposits from the urea based liquid form more dense morphologies than those from the ethylene glycol based liquid.

 Please wait while we load your content...
Please wait while we load your content...