Mn ion dissolution from MnS: a density functional theory study
Abstract
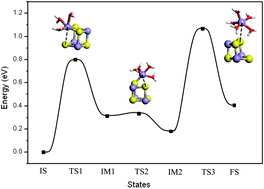
The dissolution of MnS inclusions could induce pitting corrosion in stainless steels, but its dissolution mechanism is poorly understood at the atomic scale. With the help of ab initio molecular dynamics calculations, one inevitable step in the dissolution of MnS is studied by simulating the process of one Mn ion leaving the surface. The reaction mechanism is determined to contain three steps with two large barriers and a small one, leading to two slow steps in the Mn ion dissolution. Comparing to the Na ion dissolution from NaCl, the barriers of the Mn ion dissolution are much larger, which is a reflection of their different electronic structures.

 Please wait while we load your content...
Please wait while we load your content...