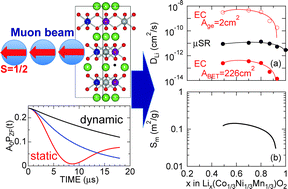
The self-diffusion coefficient of Li+ ions (DLi) in the positive electrode material Lix(Co1/3Ni1/3Mn1/3)O2 has been estimated by muon-spin relaxation (μ+SR) using powder samples with x = 1–0.49, which were prepared by an electrochemical reaction in a Li-ion battery. Here, since the implanted muons sense a slight change in the internal magnetic field due to Li-diffusion, μ+SR provides an intrinsic DLi through the temperature dependence of the nuclear field fluctuation rate (ν) [Sugiyama et al., Phys. Rev. Lett., 2009, 103, 147601]. Both DLi at 300 K and activation energy (Ea) were estimated to be ∼2.9 × 10−12 cm2 s−1 and 0.074 eV for the x = 1 sample, ∼11.0 × 10−12 cm2 s−1 and 0.097 eV for x = 0.70, and ∼8.9 × 10−12 cm2 s−1 and 0.062 eV for x = 0.49, assuming that the diffusing Li+ ions mainly jump from a regular occupied site to a regular vacant site. The estimated DLi was smaller by roughly one order of magnitude than those for LixCoO2 in the whole x range measured. Furthermore, by making comparison with DLi obtained by electrochemical measurements, the reactive surface area of the Lix(Co1/3Ni1/3Mn1/3)O2 electrode in a liquid electrolyte was found to strongly depend on x particularly at x > 0.8.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...