Tandem cathode for proton exchange membrane fuel cells
Abstract
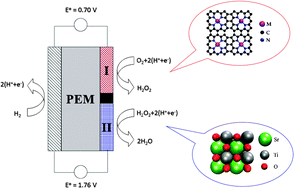
The efficiency of proton exchange membrane fuel cells is limited mainly by the oxygen reduction reaction at the cathode. The large cathodic overpotential is caused by correlations between binding energies of reaction intermediates in the reduction of oxygen to water. This work introduces a novel tandem cathode design where the full oxygen reduction, involving four electron-transfer steps, is divided into formation (equilibrium potential 0.70 V) followed by reduction (equilibrium potential 1.76 V) of hydrogen peroxide. The two part reactions contain only two electron-transfer steps and one reaction intermediate each, and they occur on different catalyst surfaces. As a result they can be optimized independently and the fundamental problem associated with the four-electron catalysis is avoided. A combination of density functional theory calculations and published experimental data is used to identify potentially active and selective materials for both catalysts. Co-porphyrin is recommended for the first step, formation of hydrogen peroxide, and three different metal oxides – SrTiO3(100), CaTiO3(100) and WO3(100) – are suggested for the subsequent reduction step.

 Please wait while we load your content...
Please wait while we load your content...