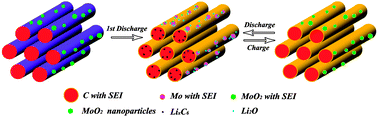
A novel hybrid of MoO2–ordered mesoporous carbon (MoO2–OMC) was prepared through a two-step solvothermal chemical reaction route. The electrochemical performances of the mesoporous MoO2–OMC hybrids were examined using galvanostatical charge–discharge, cyclic voltammetry, and electrochemical impedance spectroscopy (EIS) techniques. The MoO2–OMC hybrid exhibits significantly improved electrochemical performance of high reversible capacity, high-rate capability, and excellent cycling performance as an anode electrode material for Li ion batteries. It is revealed that the MoO2–OMC hybrid could deliver the first discharge capacity of 1641.8 mA h g−1 with an initial Coulombic efficiency of 63.6%, and a reversible capacity as high as 1049.1 mA h g−1 even after 50 cycles at a current density of 100 mA g−1, much higher than the theoretical capacity of MoO2 (838 mA h g−1) and OMC materials. The MoO2–OMC hybrid demonstrates an excellent high rate capability with capacity of ∼600 mA h g−1 even at a charge current density of 1600 mA g−1 after 50 cycles, which is approximately 11.1 times higher than that of the OMC (54 mA h g−1) materials. The improved rate capability and reversible capacity of the MoO2–OMC hybrid are attributed to a synergistic reaction between the MoO2 nanoparticles and mesoporous OMC matrices. It is noted that the electrochemical performance of the MoO2–OMC hybrid is evidently much better than the previous MoO2-based hybrids.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...