In situ SEM study of a lithium deposition and dissolution mechanism in a bulk-type solid-state cell with a Li2S–P2S5 solid electrolyte†
Abstract
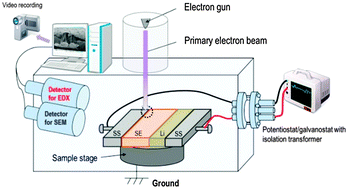
In situ SEM observation of a lithium deposition and dissolution process in an all-solid-state lithium metal battery using a sulfide-based solid electrolyte (SE) was carried out. We revealed visually that the morphology of lithium deposition varies with the operating current densities. At current densities higher than 1 mA cm−2, local lithium deposition triggers large cracks, leading to a decrease in the reversibility of lithium deposition and dissolution. On the other hand, at a low current density of 0.01 mA cm−2, its homogeneous deposition, which enables the reversible deposition and dissolution, hardly brings about the occurrence of unfavorable cracks. These results suggest that homogeneous lithium deposition on the SE and the suppression of the growth of lithium metal along the grain boundaries inside the SE are keys to achieve the repetitive lithium deposition and dissolution reaction without deterioration of the SE.

 Please wait while we load your content...
Please wait while we load your content...