From solvated ions to ion-pairing: a THz study of lanthanum(iii) hydration
Abstract
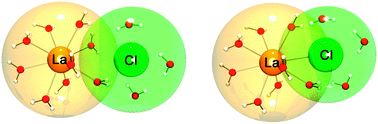
Ion radius and charge density are important parameters that determine the solvation behavior in aqueous electrolyte solutions. Here, we report on high precision THz absorption measurements of solvated LaCl3 and LaBr3 using narrow-band (75–90 cm−1) p-Ge laser and wideband (30–350 cm−1) Fourier transform

 Please wait while we load your content...
Please wait while we load your content...