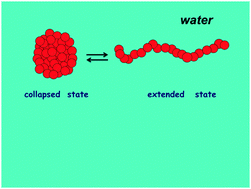
Li and Walker [Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 16527–16532] have been able to obtain the Gibbs energy change associated with the unfolding + extension of collapsed conformations of single hydrophobic chains in water, by means of atomic force microscopy measurements. These Gibbs energy changes show parabolic temperature profiles, considered to be a signature of the hydrophobic effect, and whose maximum depends on the size of monomer side chains. It has now been shown that these Gibbs energy changes can be reproduced in a more-than-qualitative manner by means of a theoretical approach developed to rationalize the temperature dependence of the conformational stability of globular proteins [G. Graziano, Phys. Chem. Chem. Phys., 2010, 12, 14245–14252]. The marked decrease in solvent-excluded volume associated with the transition from extended conformations to collapsed ones is the physical ground of the hydrophobic effect and plays the pivotal role. It is partially counterbalanced by the entropy loss upon collapse associated with the internal rotation degrees of freedom of monomer side chains. The same approach is able to account for the results obtained for the unfolding + extension of collapsed conformations of single polystyrene chains in ethanol–water solutions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...