Electrochemical characterisation of a lithium-ion battery electrolyte based on mixtures of carbonates with a ferrocene-functionalised imidazolium electroactive ionic liquid†
Abstract
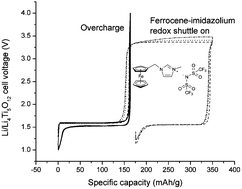
Electrolytic solutions of lithium-ion batteries can be modified with additives to improve their stability and safety. Electroactive molecules can be used as such additives to act as an electron (redox) shuttle between the two electrodes to prevent overcharging. The electroactive

 Please wait while we load your content...
Please wait while we load your content...