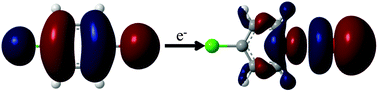
We investigated electron attachment to three dihalobenzene molecules, bromochlorobenzene (BCB), bromoiodobenzene (BIB) and chloroiodobenzene (CIB), by molecular beam photoelectron spectroscopy. The most prominent product of electron attachment in the anion mass spectra was the atomic fragment of the less electronegative halogen of the two, i.e., Br− for BCB and I− for BIB and CIB. Photoelectron spectroscopy and ab initio calculations suggested that the approaching electron prefers to attack the less electronegative atom, a seemingly counterintuitive finding but consistent with the mass spectrometric result. For the iodine-containing species BIB and CIB, the photoelectron spectrum consists of bands from both the molecular anion and atomic I−, the latter of which is produced by photodissociation of the former. Molecular orbital analysis revealed that a large degree of orbital energy reordering takes place upon electron attachment. These phenomena were shown to be readily explained by simple molecular orbital theory and the electronegativity of the halogen atoms.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...