Iron cation catalyzed reduction of N2O by CO: gas-phase temperature dependent kinetics
Abstract
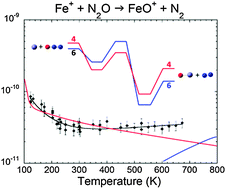
The ion–molecule reactions Fe+ + N2O → FeO+ + N2 and FeO+ + CO → Fe+ + CO2, which catalyze the reaction CO + N2O → CO2 + N2, have been studied over the temperature range 120–700 K using a variable temperature selected ion flow tube apparatus. Values of the rate constants for the former two reactions were experimentally derived as k2 (10−11 cm3 s−1) = 2.0(±0.3) (T/300)−1.5(±0.2) + 6.3(±0.9) exp(−515(±77)/T) and k3 (10−10 cm3 s−1) = 3.1(±0.1) (T/300)−0.9(±0.1). Characterizing the energy parameters of the reactions by density functional theory at the B3LYP/TZVP level, the rate constants are modeled, accounting for the intermediate formation of complexes. The reactions are characterized by nonstatistical intrinsic dynamics and rotation-dependent competition between forward and backward fluxes. For Fe+ + N2O, sextet–quartet switching of the potential energy surfaces is quantified. The rate constant for the clustering reaction FeO+ + N2O + He → FeO(N2O)+ + He was also measured, being k4 (10−27 cm6 s−1) = 1.1(±0.1) (T/300)−2.5(±0.1) in the low pressure limit, and analyzed in terms of unimolecular rate theory.

 Please wait while we load your content...
Please wait while we load your content...