The molecular clusters in a supercritical fluid–solid system should be considered as a phase—thermodynamic principle and evidence†
Abstract
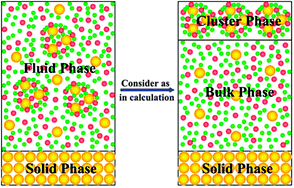
In this work we propose a new thermodynamic principle in which a supercritical fluid (SCF)–solid system is divided into a solid phase, a cluster phase, and a bulk fluid phase, i.e., the molecular clusters in the system are considered as an individual phase. The phase equilibria of various SCF–solid systems are calculated using this principle in combination with Monte Carlo simulation and the Peng–Robinson equation of state (PR-EOS). It is shown that in the critical region of the supercritical (SC)

 Please wait while we load your content...
Please wait while we load your content...