Fluorescence behavior of (selected) flavonols: a combined experimental and computational study†
Abstract
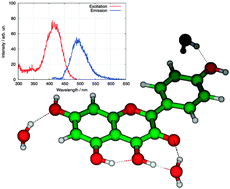
In this article, results of (time-dependent) density functional theory (DFT and TDDFT) calculations are combined with experimental absorption and fluorescence measurements to explain fluorescence properties of a series of

 Please wait while we load your content...
Please wait while we load your content...