Products of the quenching of NO A 2Σ+ (v = 0) by N2O and CO2
Abstract
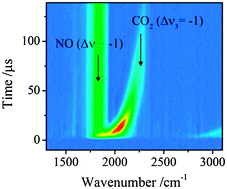
Collisional quenching of NO A 2Σ+ (v = 0) by N2O and CO2 has been studied through measurements of vibrationally excited products by time resolved Fourier transform infrared emission. In both cases vibrationally excited NO X 2Π (v) is seen and quantified in levels v ≥ 2 with distributions which are close to statistical. However the quantum yields to produce these levels are markedly different for the two quenchers. For CO2 such quenching accounts for only ca. 26% of the total: for N2O it is ca. 85%. Far more energy is seen in the internal modes of the CO2 product than those of N2O. The results are rationalised in terms of cleavage of the N2–O bond being dominant in the latter case, with either a similar O atom production or a specific channel producing almost exclusively NO in low vibrational levels (v = 0,1) for quenching by CO2. Minor reactive channels yielding NO2 are seen in both cases, and O(1D) is observed with low quantum yield in the reaction with N2O. The results are discussed in terms of previous models of the quenching processes, and are consistent with the very high yield of NO X 2Π (v = 0) previously observed by laser induced fluorescence for quenching of NO A 2Σ+ (v = 0) by CO2.

 Please wait while we load your content...
Please wait while we load your content...