Characterization of the MgO2+ dication in the gas phase: electronic states, spectroscopy and atmospheric implications†
Abstract
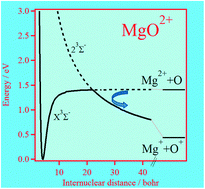
Franzreb and Williams at Arizona State University detected recently the MgO2+ molecular species in the gas phase. Here we report a very detailed theoretical investigation of the low-lying electronic states of this dication including their potentials, spin–orbit, rotational and radial couplings. Our results show that the potential energy curves of the dicationic electronic states have deep potential wells. This confirms that this dication does exist in the gas phase; it is a thermodynamically stable molecule in its ground state, and it has several excited long-lived metastable states. The potential energy curves are used then to predict a set of spectroscopic parameters for the bound states of MgO2+. We have also incorporated these potentials, rotational and radial couplings in dynamical calculations to derive the cross sections for the charge transfer Mg2+ + O → Mg+ + O+ reaction in the 1–103 eV collision energy domain via formation–

 Please wait while we load your content...
Please wait while we load your content...