Resonance-assisted hydrogen bonds revisited. Resonance stabilization vs. charge delocalization†
Abstract
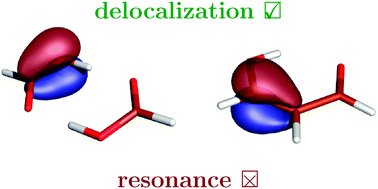
The origins of stabilization in the short strong hydrogen bonds commonly referred to as “resonance-assisted” (RAHB) have been revisited using the modern valence-bond theory, the hybrid variational–perturbational interaction energy

 Please wait while we load your content...
Please wait while we load your content...