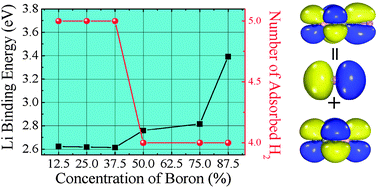
A balance between the hydrogen capacity and reversibility is a big challenge in the search for hydrogen storage materials. Using van der Waals-corrected density functional theory, we perform a detailed study of the hydrogen molecules adsorption on lithium (Li) decorated zero- and two-dimensional boron–carbon (B–C) compounds. It is found that not only the Li bond strength but also the number of adsorbed hydrogen molecules depends on the B concentration. First, the binding of Li on the B–C compounds strengthens with the increase of the B concentration due to the stronger hybridization between the lowest unoccupied molecular orbitals of the B–C compounds and Li 2p orbitals. Thus, Li atoms are not likely to form clusters, indicating a good reversible hydrogen storage. Second, higher B concentration results in weaker electric field produced by the charge transfer from Li to the B–C compounds. Therefore, one Li atom can adsorb up to 5H2 molecules with the B concentration less than 50%. In contrast, the adsorption number of H2 molecules is reduced to 4 when the B concentration is greater than or equal to 50%. Third, using a statistical model parametrized by the results of ab initio calculations, the adsorption and desorption of molecular hydrogens are calculated at ambient temperature and pressure. We find that the usable number of adsorbed H2 per Li under ambient conditions decreases with the increase of B concentration. These results can serve as a guide in the design of new hydrogen storage materials based on B–C compounds.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...