Do primary nitrosamines form and exist in the gas phase? A computational study of CH3NHNO and (CH3)2NNO†
Abstract
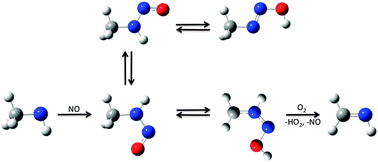
The reactions of the CH3NH and (CH3)2N radicals with NO have been studied using quantum chemistry methods to compare the formation and stability of primary and secondary ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH on a short timescale. TDDFT, CASPT2 and MR-CI calculations show little difference between the n → π* transitions in CH3NHNO and (CH3)2NNO and that the two molecules should have comparable photolysis lifetimes in the atmosphere.
NH on a short timescale. TDDFT, CASPT2 and MR-CI calculations show little difference between the n → π* transitions in CH3NHNO and (CH3)2NNO and that the two molecules should have comparable photolysis lifetimes in the atmosphere.

 Please wait while we load your content...
Please wait while we load your content...