Nitrogen defects in wide band gap oxides: defect equilibria and electronic structure from first principles calculations
Abstract
The 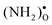 as well as
as well as 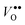 and
and 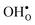 in MgO, CaO, SrO, Al2O3, In2O3, Sc2O3, Y2O3, La2O3, TiO2, SnO2, ZrO2, BaZrO3, and SrZrO3. The NqO acceptor level is found to be deep and the binding energy of NH×O with respect to
in MgO, CaO, SrO, Al2O3, In2O3, Sc2O3, Y2O3, La2O3, TiO2, SnO2, ZrO2, BaZrO3, and SrZrO3. The NqO acceptor level is found to be deep and the binding energy of NH×O with respect to 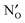 and
and 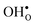 is found to be significantly negative, i.e. binding, in all of the investigated
is found to be significantly negative, i.e. binding, in all of the investigated 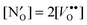 predominates at higher temperatures (>900 K for most of the
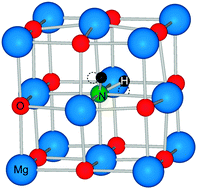
predominates at higher temperatures (>900 K for most of the 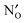 is found to introduce isolated N-2p states within the band gap, while the N-2p states of NH×O are shifted towards, or overlap with the VBM. Finally, we assess the effect of
is found to introduce isolated N-2p states within the band gap, while the N-2p states of NH×O are shifted towards, or overlap with the VBM. Finally, we assess the effect of

 Please wait while we load your content...
Please wait while we load your content...