Intramolecular hydrogen atom migration along the backbone of cationic and neutral radical tripeptides and subsequent radical-induced dissociations†
Abstract
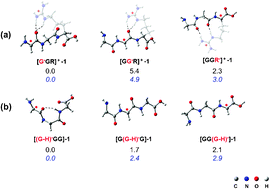
Dissociation of peptide radical ions involves competition between charge-induced and radical-induced reactions that can be preceded by isomerization. The isomeric radical cations of the peptide methyl ester [G˙GR–OMe]+ and [GG˙R–OMe]+ provide very similar collision-induced dissociation (CID) spectra, suggesting that isomerization occurs prior to fragmentation. They undergo characteristic radical-induced bond cleavage of the peptide N-terminal amide bond resulting in the y2+ ion, and of the arginine side-chain's Cα–Cβ bond giving protonated allylguanidine {[CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2NHC(NH2)2]+, m/z 100}. The absence of a y2+ fragment ion in the CID of the radical cationic tripeptide [ACH3G˙R]+ and of an m/z 100 ion in the spectrum of [G˙ACH3R]+ (where ACH3 is an α-aminoisobutyric acid residue, which cannot form an α-carbon-centered radical through hydrogen atom transfer) establishes the importance of hydrogen atom migration along the peptide backbone prior to specific radical-induced fragmentations. Herein we use density functional theory (DFT) at the B3LYP/6-31++G(d,p) level to evaluate the barriers for interconversion between the α-carbon-centered radicals and for dissociation. The radical cations [G˙GR]+ and [GG˙R]+ have their radicals located on the α-carbon atoms of the peptide backbone and their charge densities largely sequestered on the guanidine groups of the side-chain of arginine residues. This is in contrast to the isomeric radical cations of [GGG]˙+, in which the charge resides necessarily on the peptide backbone. The lower charge densities on the backbones of [G˙GR]+ and [GG˙R]+ result in greater structural flexibility, decreasing the barrier for interconversion between these α-carbon-centered radicals to 36.2 kcal mol−1 (cf. 44.7 kcal mol−1 for [GGG]˙+). The total absence of charge, assessed by examining intramolecular hydrogen atom transfers among the three α-carbon centers of the isomeric neutral α-carbon-centered triglycine radicals [GGG–H]˙, leads to an additional but slight reduction in enthalpy, to approximately 34 kcal mol−1.
CHCH2NHC(NH2)2]+, m/z 100}. The absence of a y2+ fragment ion in the CID of the radical cationic tripeptide [ACH3G˙R]+ and of an m/z 100 ion in the spectrum of [G˙ACH3R]+ (where ACH3 is an α-aminoisobutyric acid residue, which cannot form an α-carbon-centered radical through hydrogen atom transfer) establishes the importance of hydrogen atom migration along the peptide backbone prior to specific radical-induced fragmentations. Herein we use density functional theory (DFT) at the B3LYP/6-31++G(d,p) level to evaluate the barriers for interconversion between the α-carbon-centered radicals and for dissociation. The radical cations [G˙GR]+ and [GG˙R]+ have their radicals located on the α-carbon atoms of the peptide backbone and their charge densities largely sequestered on the guanidine groups of the side-chain of arginine residues. This is in contrast to the isomeric radical cations of [GGG]˙+, in which the charge resides necessarily on the peptide backbone. The lower charge densities on the backbones of [G˙GR]+ and [GG˙R]+ result in greater structural flexibility, decreasing the barrier for interconversion between these α-carbon-centered radicals to 36.2 kcal mol−1 (cf. 44.7 kcal mol−1 for [GGG]˙+). The total absence of charge, assessed by examining intramolecular hydrogen atom transfers among the three α-carbon centers of the isomeric neutral α-carbon-centered triglycine radicals [GGG–H]˙, leads to an additional but slight reduction in enthalpy, to approximately 34 kcal mol−1.

 Please wait while we load your content...
Please wait while we load your content...