Amyloid-β fibril disruption by C60—molecular guidance for rational drug design†
Abstract
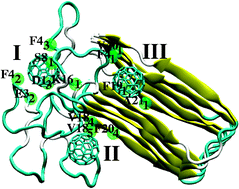
The WHO has listed Alzheimer's disease among the major neurological disorders with an estimated 35 million people affected worldwide. Amyloid-β is mostly believed to be the causative factor in Alzheimer's disease and the severity of the disease correlates with the tendency of amyloid-β to form aggregation patterns—plaques. Lacking effective medication, the identification of any underlying mechanistic principles regarding plaque formation appears to be crucial. Here we carry out computer simulations to study the effect of C60 on structure and stability of an idealised pentameric construct of amyloid-β units (a model fibril). A binding site on top of the structurally ordered stack of β-sheets is identified that triggers structural alterations at the turn region of the hook-like β-sheet

 Please wait while we load your content...
Please wait while we load your content...