Dissociative and non-dissociative adsorption dynamics of N2 on Fe(110)
Abstract
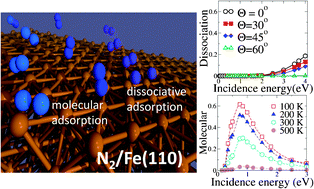
We study the adsorption dynamics of N2 on the Fe(110) surface. Classical molecular dynamics calculations are performed on top of a six-dimensional potential energy surface calculated within density functional theory. Our results show that N2 dissociation on this surface is a highly activated process that takes place along a very narrow reaction path with an energy barrier of around 1.1 eV, which explains the measured low reactivity of this system. By incorporating energy exchange with the lattice in the dynamics, we also study the non-dissociative molecular

 Please wait while we load your content...
Please wait while we load your content...