Hofmeister series reversal for lysozyme by change in pH and salt concentration: insights from electrophoretic mobility measurements†
Abstract
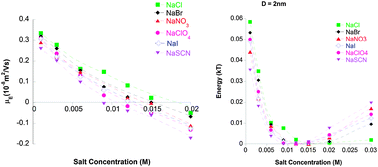
Hofmeister series reversal can occur with change in pH, or increase in salt concentration. The phenomena are a challenge for any theory of ion specific effects. Recent theoretical work predicts how a complex interplay between ionic sizes, hydration and dispersion forces explains Hofmeister series reversal. Electrophoretic mobility measurements on lysozyme suspensions reported here are consistent with the theory.

 Please wait while we load your content...
Please wait while we load your content...