A comparative study on Na2MnPO4F and Li2MnPO4F for rechargeable battery cathodes†
Abstract
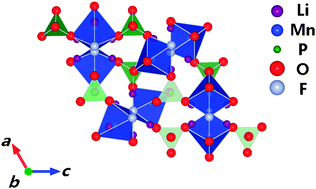
Electrochemical properties of A2MnPO4F (A = Na/Li) were investigated both experimentally and with first principles calculations. A new Li2MnPO4F phase was successfully synthesized for the first time. A one

 Please wait while we load your content...
Please wait while we load your content...