Temperature-responsive ionic liquid/water interfaces: relation between hydrophilicity of ions and dynamic phase change
Abstract
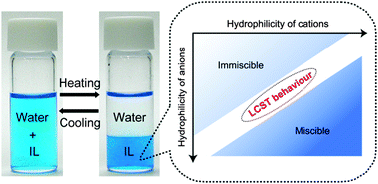
Phase separation between ionic liquids (ILs) and molecular liquids is of interest physico-chemically, and also has industrial relevance. IL/water mixtures are of great interest in many fields. Unlike static phase separation between IL and water, dynamic shifts of IL/water mixtures between a homogeneous mixture and separate phases have a wide variety of applications. The miscibility of ILs with water generally increases upon heating, and a few ILs undergo a lower critical solution temperature (LCST)-type phase transition with water in which the separated biphases become miscible upon cooling. As the phase transition is controlled by changing the temperature by a few degrees, the LCST-type phase response of IL/water mixtures makes it possible to use ILs as solvents in various energy-saving processes. Since many hydrophilic ILs do not undergo phase separation with water, we aim to determine the necessary conditions under which hydrophobic ILs undergo the phase transition. Based on physico-chemical analysis of many hydrophobic ILs that undergo a phase separation after mixing with water, we find there is a particular range of “hydrophilicity” of these hydrophobic ILs within which the LCST-type phase transition is possible. Accordingly, a hydrophilicity index (HI) of ILs is proposed, in terms of the number of water molecules in the separated IL phase. The HI value proves to be a good indicator of the phase behaviour of IL/water mixtures, as well as their phase transition temperature. Potential application of the LCST-type phase change to the selective extraction of water-soluble proteins is also summarised.
- This article is part of the themed collection: Interfaces of Ionic Liquids

 Please wait while we load your content...
Please wait while we load your content...