Protein binding on stepped calcite surfaces: simulations of ovocleidin-17 on calcite {31.16} and {31.8}†
Abstract
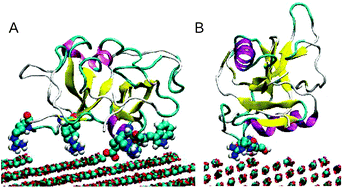
Simulations using classical molecular dynamics are reported on the binding of the protein Ovocleidin-17 to calcite stepped surfaces. vicinal surfaces ({31.8} and {31.16}) are used to obtain acute and obtuse steps. The simulations demonstrate that binding is greater at the obtuse step. A range of analytical methods is used to show the importance of surface and local water structure for protein binding. We discuss the general features of molecular binding in the light of these results. Our analysis shows that it is unlikely that Ovocleidin-17 is important in controlling crystal morphology; its main role is likely to be in controlling calcite nucleation.

 Please wait while we load your content...
Please wait while we load your content...