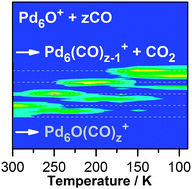
The gas phase reactions of carbon monoxide with small mass-selected clusters of palladium, Pdx+ (x = 2–7), and their oxides, PdxO+ (x = 2–7) and PdxO2+ (x = 4–6), have been investigated in a radio frequency ion trap operated under multi-collision conditions. The bare palladium clusters were found to readily adsorb CO yielding a highly size dependent product pattern. Most interestingly, the reactions of the pre-oxidized palladium clusters with CO lead to very similar product distributions of Pdx(CO)z+ complexes as in the case of the corresponding pure Pdx+ clusters. Consequently, it has been concluded that the investigated palladium oxide clusters efficiently oxidize CO under formation of the bare clusters, which further adsorb CO molecules yielding the previously observed Pdx(CO)z+ product complex distributions. This CO combustion reaction has been observed even at temperatures as low as 100 K. However, for Pd2O+, Pd6O+, Pd6O2+, and Pd7O+ a competing reaction channel yielding palladium oxide carbonyls PdxO(CO)z+ could be detected. The latter adsorption reaction may even hamper the CO combustion under certain reaction conditions and indicates enhanced activation barriers involved in the CO oxidation and/or the CO2 elimination process on these clusters.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...