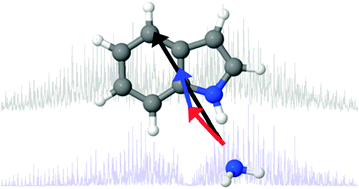
Experimentally measured permanent dipoles induced by hydrogen bonding. The Stark spectrum of indole–NH3†
Abstract
Hydrogen bond pairs involving the
- This article is part of the themed collection: Hydrogen bonding in electronically excited states

 Please wait while we load your content...
Please wait while we load your content...