Hydrogen bond effects in the ground and excited singlet states of 4H-1-benzopyrane-4-thione in water—theory and experiment†
Abstract
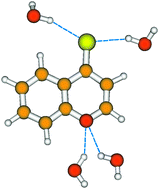
The hydrogen bond energies for 4H-1-benzopyrane-4-thione (BPT) in its ground and two lowest excited singlet electronic states have been determined using ab initio methods. It was shown that the BPT molecule can form, as an acceptor, four relatively strong hydrogen bonds with water molecules, leading to a stable complex in the ground electronic state S0. The hydrogen bonds involving the sulfur atom from the thiocarbonyl group were found to be stronger than those involving the oxygen atom from the benzopyran moiety. The former hydrogen bonds were predicted to become significantly weaker upon excitation to the S1 state and, in contrast, stronger upon excitation to the S2 state. Calculated changes in the hydrogen bond energy due to the S0 → S1 and S0 → S2 excitation are in very good agreement with the experimental values obtained from the absorption solvatochromic study, according to a procedure proposed by us in [E. Krystkowiak, et al., J. Photochem. Photobiol. A: Chem., 2006, 184, 250]. The maxima of absorption spectra of the BPT–water hydrogen-bonded complex, calculated taking into consideration nonspecific solute–solvent interactions, are also in good agreement with the experimental results.
- This article is part of the themed collection: Hydrogen bonding in electronically excited states

 Please wait while we load your content...
Please wait while we load your content...