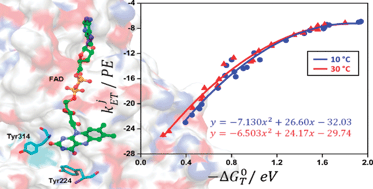
The structural basis for the temperature-induced transition in the D-amino acid oxidase (DAAO) monomer from pig kidney was studied by means of molecular dynamic simulations (MDS). The center to center (Rc) distances between the isoalloxazine ring (Iso) and all aromatic amino acids (Trp and Tyr) were calculated at 10 °C and 30 °C. Rc was shortest in Tyr224 (0.82 and 0.88 nm at 10 and 30 °C, respectively), and then in Tyr228. Hydrogen bonding (H-bond) formed between the Iso N1 and Gly315 N (peptide), between the Iso N3H and Leu51 O (peptide) and between the Iso N5 and Ala49 N (peptide) at 10 °C, whilst no H-bond was formed at the Iso N1 and Iso N3H at 30 °C. The H-bond of Iso O4 with Leu51 N (peptide) at 10 °C switched to that with Ala49 N (peptide) at 30 °C. The reported fluorescence lifetimes (228 and 182 ps at 10 and 30 °C, respectively) of DAAO were analyzed with Kakitani and Mataga (KM) ET theory. The calculated fluorescence lifetimes displayed an excellent agreement with the observed lifetimes. The ET rate was fastest from Tyr224 to the excited Iso (Iso*) at 10 °C and from Tyr314 at 30 °C, despite the fact that the Rc was shortest between Iso and Tyr224 at both temperatures. This was explained by the electrostatic energy in the protein. The differences in the observed fluorescence lifetimes at 10 and 30 °C were ascribed to the differences in electron affinity of the Iso* at both temperatures, in which the free energies of the electron affinity of Iso* at 10 and 30 °C were −8.69 eV and −8.51 eV respectively. The other physical quantities related to ET did not differ appreciably at both temperatures. The electron affinities at both temperatures were calculated with a semi-empirical molecular orbital method (MO) of PM6. Mean calculated electron affinities over 100 snapshots with 0.1 ps intervals were −7.69 eV at 10 °C and −7.59 eV at 30 °C. The difference in the calculated electron affinities, −0.11 eV, was close to the observed difference in the free energies, −0.18 eV. The present quantitative analysis predicts that the highest ET rate can occur from a donor with longer donor–acceptor distance, which was explained by differences in electrostatic energy.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...