DFT investigation of NH3 physisorption on CuSO4 impregnated SiO2†
Abstract
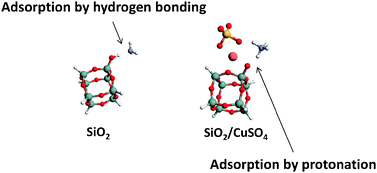
In this quantum chemical investigation, NH3 physisorption onto a model of copper sulfate impregnated silica is compared with pure silica and copper sulfate adsorbents. The physisorption process is modeled as direct binding of the NH3 molecule to the adsorption site of the dry adsorbents and as displacement of a H2O molecule by NH3 in the hydrated complexes. The surface of silica is represented by a hydroxyl group attached to a silsesquioxane cage, H7Si8O12(OH) and silica impregnated with CuSO4 by the most stable configuration of the cluster containing a CuSO4 ion pair placed adjacent to the silica cage. H2O is systematically added to the dehydrated adsorbents to investigate the role of water in NH3 adsorption. Modeling hydrated environments of each type of adsorbent is focused on H2O molecules that directly coordinate with the active sites. The results indicate that the binding energy of adsorbing NH3 onto the mixed adsorbent is greater than in pure silica. This enhanced binding in the mixed adsorbent is consistent with improved Brønsted acidity of the silanol in the presence of CuSO4.

 Please wait while we load your content...
Please wait while we load your content...