Throwing jellium at gallium—a systematic superatom analysis of metalloid gallium clusters
Abstract
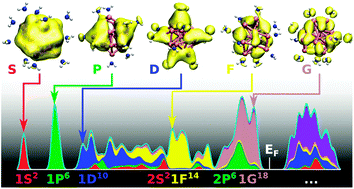
Motivated by recent developments in the field of so-called “superatom complexes”, as well as by the challenge posed to theory in understanding the many polymorphs of gallium, we analyse the electronic structure of several previously synthesised

 Please wait while we load your content...
Please wait while we load your content...